Oct 23,2024
Mole Day is an unofficial holiday celebrated annually on October 23 from 6:02 am to 6:02 pm. This date and time honour Avogadro’s Number, which is a basic concept in chemistry. The day promotes interest in chemistry, especially among students of this particular branch, educators and scholars.
The concept of Mole Day was conceived in the early 1980s by then-National Science Teacher Association (NSTA) member Margaret Christoph of Prairie du Chien, Wisconsin.
In an article in The Science Teacher, Christoph discussed the significance of a mole in chemistry. Following this, Maurice Oehler, a high school chemistry teacher in Wisconsin, established the National Mole Day Foundation (NMDF) on May 15, 1991. The foundation aims to inspire the students to learn chemistry through educational activities.
Mole Day aims to spark interest in chemistry by engaging students in experiments, projects, and games centered around mole concepts. These activities are designed to make learning more fun and interactive, often with a specific theme to guide the exploration.
Knowledge of these attributes is essential for anyone pursuing a course in Science, Technology, Engineering or Mathematics (STEM).
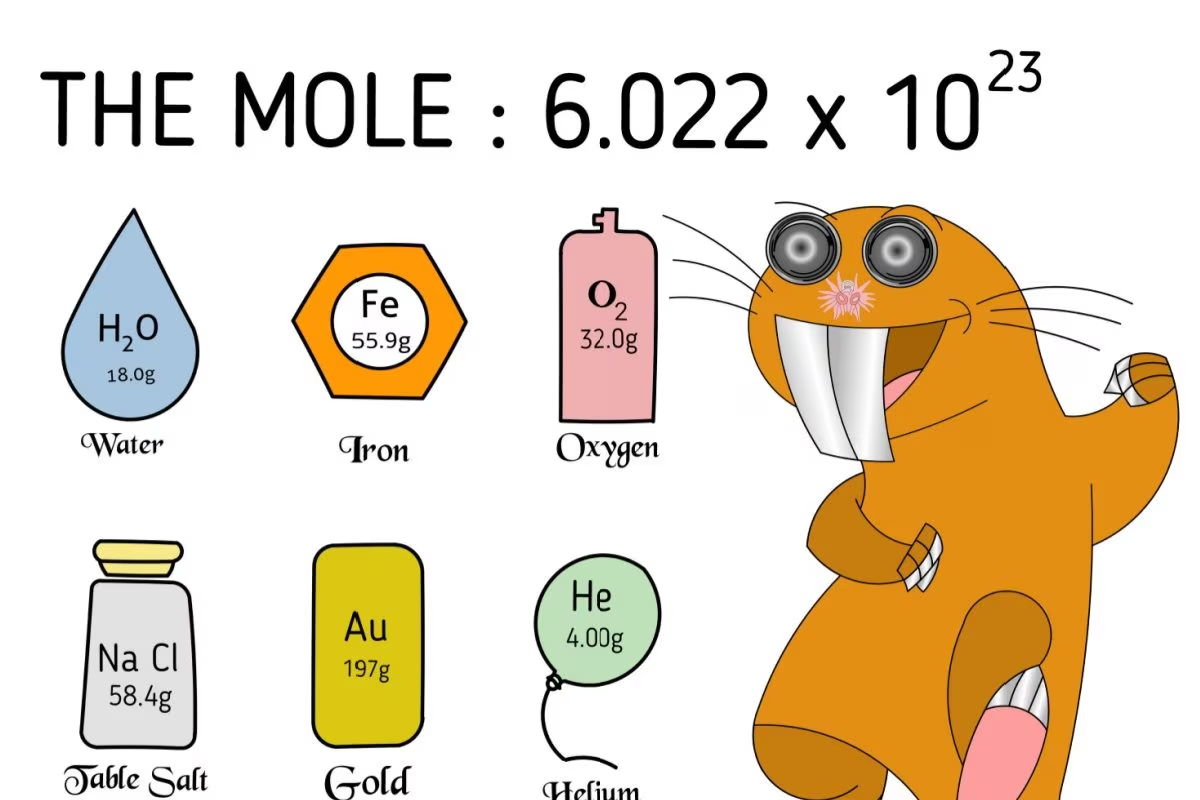
A mole is a unit that measures an amount of substance. It is important because the properties of materials at the nanoscale are important for explaining their behaviour at the atomic scale and at the macro level. For instance, you want to determine the precise number of marbles in the heap without having to count each marble one at a time. That is where chemists use Avogadro’s number, or mole.
Avogadro’s number is a very large number, which is 6.02 x 10²³ or 602 with 21 zeros after it. It determines the number of particles in the given substance, that is in one mole of any particular substance.
Avogadro’s number is just a way of telling how many tiny particles there are in a substance through weight.
The idea that would eventually become Avogadro’s Number was first posited by an Italian scientist named Amedeo Avogadro in 1811. Avogadro theorised that equal volumes of gases at the same temperature and pressure contain the same number of molecules.
But it would take another few decades for scientists to begin making exact measurements with the aid of experiments that would measure movements like Brownian motion, conducted by Robert Brown in 1827, which later helped produce a more accurate value for Avogadro’s Number.
.svg)
